- 爱妻自媒体-微信公众平台文章推荐

Changing our microbiome: probiotics in dermatology [原文来自:www.ii77.com]
Y. Yu¹, S. Dunaway¹, J. Champer², J. Kim³ and A. Alikhan⁴
1.Department of Dermatology, University of Cincinnati, Cincinnati, OH, U.S.A.
2.Department of Computational Biology and Department of Molecular Biology and Genetics, Cornell University, Ithaca, NY, U.S.A.
3.Division of Dermatology and Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA, U.S.A.
4.Sutter Medical Foundation, Sacramento, CA, U.S.A.
提纲
配景:在人类健康与发病机制中,共生细菌饰演着主要的脚色。人类对共生细菌的乐趣一日千里,且不光仅局限于胃肠道微生物菌群,还包罗皮肤微生物菌群以及其对多种皮肤疾病的影响。
目的:本文综述与肠道和皮肤微生物菌群在皮肤病的应用相关的最新进展,评估口服或外用益生菌对治疗某些皮肤病是否有效的相关临床数据。
方式:采用PubMed和ClinicalTrials.gov数据库完成与根蒂科学、转化研究和临床研究相关的搜刮,说明使用益生菌前后皮肤微生态的差别以及其对特应性皮炎、平常痤疮、银屑病、难愈创面、脂溢性皮炎和皮肤肿瘤的影响。
究竟:除特应性皮炎外,很少有临床试验研究益生菌在皮肤病防治中的应用。且大部门研究都集中在口服益生菌干涉以及局部施用益生菌两方面,在皮肤共生菌方面的研究还较为缺乏。总之,现有的临床试验获得了较为积极的究竟:采用益生菌干涉有助于改善皮肤状况。
结论:口服和局部使用益生菌对于治疗某些炎性皮肤病显露出积极结果,在创面修复和皮肤癌治疗方面也显露潜力,但仍需更多研究来验证。
已知信息:
1. 微生物菌群在人类健康和发病机制中施展着主要的感化。
2. 益生菌有助于调节宿主的微生物菌群,并或者有利于患者的健康。
3. 迄今已开展口服或局部外用益生菌对特定皮肤病疗效的索求。
本研究的立异点:
1. 从根蒂科学与临床试验数据综述了肠道与皮肤微生物菌群在皮肤病学中的感化。
2. 综述益生菌防治包罗特应性皮炎、平常痤疮、银屑病、脂溢性皮炎、难愈创面以及皮肤肿瘤在内的皮肤病最新资料。
3. 提出了将来的益生菌干涉方案。
共生细菌对于维持人类健康的免疫系统至关主要,菌群失调有利于机会致病菌以及致病细菌的定植,导致疾病的发生。已有大量文献报道与胃肠道疾病相关的微生物菌群,以及其对皮肤的影响¹ ²。固然定植于皮肤上的微生物数量显著低于胃肠道菌群,但二者对免疫系统的调节及其致病机理都较为相似³。
微生物菌群在疾病中所饰演的脚色已经被研究得越来越透辟⁴,是以经由微生物调节人体免疫系统来治疗相关疾病这一新方式具有广宽的应用前景。个中一种直接的方式就是包罗活菌在内的微生物制剂(益生菌)的使用⁵,当其达到充沛浓度时,将有利于宿主的健康⁶。本文综述微生物菌群对人类健康的影响,稀奇是口服和局部外用益生菌在皮肤病防治中是若何施展感化的。
方式
以“微生物组”、“微生物菌群”、“共生”、“细菌”、“微生物制剂”、“局部的”、“皮肤”和“皮肤病学”作为要害词,在PubMed和ClinicalTrial.gov数据库搜刮查找相关研究文献。将所有的检索词做分歧组合,并依据文献摘要的相关性进行筛选。经由根蒂科学、体外试验、动物模型研究以及临床试验进行口服和局部外用益生菌干涉的研究(本综述未包罗非英文相关文献)。重点是已经研究发现皮肤微生物组学有差别的皮肤病,包罗特应性皮炎(atopic dermatitis, AD)、平常痤疮、银屑病、脂溢性皮炎、难愈创面及伤口愈合、皮肤肿瘤,以及益生菌在这些患者群体中的影响。
皮肤微生态与局部外用益生菌
人体皮肤定植有跨越1000种细菌,每一种都能与特定的情况相适应⁷。遍及来讲,大部门皮肤细菌都是对宿主无害的共生细菌,组成了皮肤微生物菌落的多样性,有助于预防或缓解疾病(图1)⁸⁻¹⁰。共生细菌经由被动占有和致病微生物相似的生态位干扰致病微生物定植,还经由排泄抗菌因子与致病菌自动竞争。共生细菌还能调节免疫系统,指导免疫系统冲击致病微生物或许提高免疫耐受力,从而缓解炎症¹¹ ¹²。
局部外用微生物制剂是改变皮肤微生物菌群以及对多种疾病进行免疫应答的一种直接方式⁸ ⁹。固然现阶段相关研究较少,但一些局部外用益生菌的临床试验已经显现出良性的究竟。
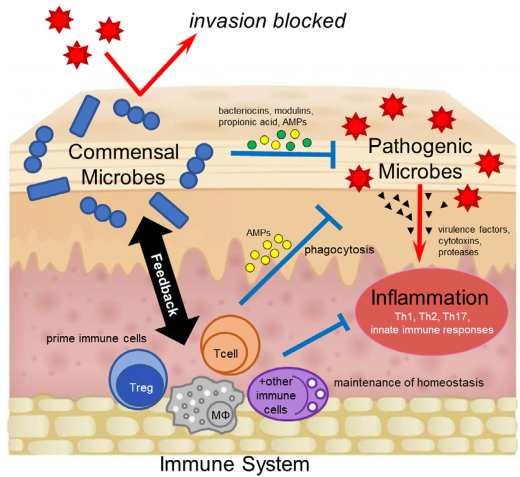
图1 共生细菌按捺疾病的机制。共生细菌平日和致病微生物占有沟通的生态位,直接故障致病菌定植。共生菌也或者直接排泄如细菌素、丙酸、抗菌肽(AMPs)等或间接调节免疫系统来冲击致病菌。
肠道微生物菌群和口服益生菌与皮肤病
肠道微生物菌群诱发和预防疾病的相关机制在皮肤方面有普遍应用。正常肠道菌群失调会促进慢性炎症的发生,甚至导致肿瘤¹³ ¹⁴。肠道共生菌可以自力调节懈弛解胃肠道炎症¹³。是以,共生菌在健康肠道微生物菌群重建过程中有抗氧化感化,诱导肿瘤按捺基因表达¹⁵,激发免疫系统冲击癌细胞,或经由调控T细胞来削减炎症发生¹⁶⁻¹⁸,匡助宿主抵当恶性肿瘤。
同时,肠道微生物可以影响系统性炎症、氧化应激、血糖掌握以及组织脂质含量²。肠道菌群失调与包罗和肠道相关的皮肤病-关节炎综合症² ¹⁹以及玫瑰痤疮²⁰在内的炎症性皮肤病具有相关性:经由重建正常的肠道微生物菌群使得整个疾病获得缓解。是以,口服益生菌能够调节肠道微生物菌群,从而间接影响皮肤病。其相关治疗在特应性皮炎的研究最透辟。
特应性皮炎(Atopic dermatitis, AD)
特应性皮炎(AD)患者的表皮屏障功能障碍和免疫失调,有利于致病细菌在其体内定植。AD型皮肤外观金..葡萄球菌(Staphylococcus aureus)的数量增多,而整体的微生物多样性削减²¹⁻²⁴。最新研究证实无论是AD患者的皮损部位照样非皮损部位菌群多样性都低于健康人,即AD患者全身的皮肤微生物菌群都发生了改变²⁵。同时,AD的严重水平与微生物多样性成负相关。当患者病情严重时,能视察到皮肤微生物菌群的多样性急剧削减,而当对AD皮损部位进行治疗时,微生物菌群多样性又得以重建²¹ ²⁴ ²⁵。稀奇地,葡萄球菌属的种类在AD活跃期有所增加,个中包罗表皮葡萄球菌(Staphylococcus epidermis),这或者是掌握金..葡萄球菌数量的一种代偿机制²¹ ²² ²⁴。切实,一项针对AD活跃期的查询显露:金..葡萄球菌在重型患者中占主导优势,而表皮葡萄球菌在相对不严重患者中占主导²⁶。并且,肠道情况也会影响AD的致病菌。患有与IgE相关湿疹的婴幼儿,体内双歧杆菌的种类削减,且微生物多样性低于健康婴幼儿¹⁹。2月龄时,大肠杆菌(Escherichia coli)在婴儿肠道中定植,有利于婴儿更长久的健康,好比,其能降低6岁儿童AD的发病率²⁷。是以,调节微生物菌群在AD的治疗中是一种前景广宽的新策略。
口服益生菌在防治AD方面的疗效已经进行了大型排队研究和随机对照试验。最新的调集剖析究竟显露,口服发酵乳酸杆菌(Lactobacillus fermentum)、唾液乳酸杆菌(Lactobacillus salivarius)以及分歧菌种的夹杂物后,1070名儿童AD患者的AD积分指数值(SCORAD)显著降低²⁸。早前的调集剖析究竟²⁹也支撑口服益生菌可以防治AD:无论是零丁服用乳酸杆菌照样乳酸杆菌与双歧双歧杆菌(Bifidobacterium bifidum)结合服用,都能阻止AD病情成长,其优势比离别为0.7和0.62³⁰。此外,病情适中的AD患者每日口服双歧杆菌和干酪乳酸杆菌(Lactobacillus casei)的夹杂物,一连服用12周后,与不采用益生菌干涉的对照组比拟,其SCORAD值平均降低19.2分³¹。然而,疗效也与菌种特异性相关,好比患者口服鼠李糖乳酸杆菌(Lactobacillus rhamnosus)和副干酪乳杆菌(Lactobacillus paracasei)后得出了分歧的究竟³²⁻³⁶。
迄今为止,仍很少有研究报道局部施用微生物制剂进行AD治疗。Nakatusji等近期报道下场部外用皮肤共生菌来匹敌致病菌的相关案例³⁷。将皮肤共生菌—凝固酶阴性的葡萄球菌接种至AD患者的皮肤外观,因为该共生菌会排泄高效的抗菌肽,可以选择性地杀死金..葡萄球菌,从而导致其菌落数量削减³⁷。早期数据显露,凝固酶阴性葡萄球菌干涉不光可以按捺金..葡萄球菌的生长,还与临床症状改善以及缓解局部炎症相关³⁸。此外,与使用糖皮质激素比拟,局部接种革兰阴性菌玫瑰单胞菌(Roseomonas mucosa)于成人和儿童AD患者皮肤上后,患者瘙痒症状显着减轻,SCORAD值降低,且无副感化和并发症发生³⁹。
此外,一些关于肠道共生菌的局部使用报道也收获可喜究竟。接种约氏乳酸杆菌(Lactobacillus johnsonii)至AD皮损部位(一天2次,3周)时,金..葡萄球菌载量降低,直接导致SCORAD值降低⁴⁰。另一篇研究报道,在AD患者皮损处局部涂抹2周嗜热链球菌(Streptococcus thermophilus)乳霜,患者皮肤红斑,鳞屑以及瘙痒症状都获得了显着的改善⁴¹。将含有革兰阴性菌线状透亮颤菌(Vitreoscilla filiformis,在温泉水中被发现)裂解物的乳霜涂抹于AD患处时,也可以使AD患者的临床症状获得改善⁴²。固然益生菌疗法在AD治疗的研究才方才起步,但好多研究都呈现出了阳性的究竟。
平常痤疮(Acne vulgaris)
平常痤疮与痤疮丙酸杆菌(Cutibacterium acnes,由Propionibacterium acnes改名而来)直接相关。肠道微生物菌群失调可以经由肠-皮轴影响痤疮的发病²,是以口服益生菌对于治疗痤疮具有潜在疗效。研究表明,嗜酸乳杆菌(Lactobacillus acidophilus)、保加利亚乳杆菌(Lactobacillus delbrueckii bulgaricus)以及双歧双歧杆菌在治疗痤疮方面的结果与使用二甲胺四环素相当,且副感化更小,口服益生菌12周后,痤疮患者皮损削减67%⁴³。二甲胺四环素与益生菌结合使用时,治疗结果更佳⁴³。另一项研究表明,痤疮患者在一连服用保加利亚乳杆菌和嗜热链球菌12周后,患者的炎性皮损削减30%,其皮脂含量和游离脂肪酸含量与对照组比拟降低了50%或更多⁴⁴。近期研究发现,与口服抚慰剂的对照组(胰岛素旌旗基因可以正常表达)比拟,患者一连12周口服鼠李糖乳酸杆菌SP1后,其背部痤疮获得了显着改善⁴⁵。
近期研究发现,痤疮丙酸杆菌的某些特定亚型(IA-2,IB-1,I-C)与痤疮更相关,而其他亚型(II-核酸型6,III)多显现在健康皮肤上⁴⁶⁻⁵⁰。是以,这些与痤疮相关的亚型很或者是导致痤疮发生的身分。一项体外实验究竟显露,与痤疮相关的痤疮丙酸杆菌亚型能诱导T辅助细胞(Th)1和Th17的高响应;但与健康皮肤相关的亚型导致(Th)1和Th17低响应,白介素(IL)-10抗炎症高响应⁵¹。以上究竟解说,与健康皮肤相关的痤疮丙酸杆菌亚型或者用于局部益生菌防治痤疮中,用于替代痤疮相关的细菌亚型以及其他潜在的机会致病细菌亚型⁴⁶ ⁵¹ ⁵²。以上靶向策略就是行使同种的细菌占有统一生态位,是以比选择其他皮肤或肠道共生菌加倍高效,可以快速和持久的改善皮肤微生态。
然而,使用肠道和其他非皮肤益生菌治疗痤疮的相关研究还很有限。痤疮患者在一连使用从粪肠球菌(Enterococcus faecalis)SL-5中提取的促肠菌素冻干粉8周后,其炎性皮损与对照组比拟,下降了60%⁵³。一项针对亚硝化单胞菌(Nitrosomonas eutropha)的新型IIb/III的研究报道显露,与对照组比拟,实验组在评估痤疮严重水平的研究者总体评分(Investigator’s Global Assessment)中下降2点,且呈现出炎性皮损数量削减的趋势(一天2次,一连使用12周)⁵⁴。但这些菌并不是皮肤上天然定植,是以当完结治疗时,就不太或者像皮肤微生物一般,为患者供应持续珍爱。比拟而言,一周2次局部涂抹含有表皮葡萄球菌的凝胶,改变了宿主的皮肤微生物菌群,且表皮葡萄球菌可以很好地定植于皮肤上⁵⁵。是以在痤疮治疗中,选择像表皮葡萄球菌如许的皮肤共生菌比亚硝化单胞菌更合适,尤其是体外试验显露表皮葡萄球菌可以按捺痤疮丙酸杆菌生长⁵⁶。
局部外用益生菌也包含痤疮丙酸杆菌噬菌体(可以裂解宿主细菌的病毒)。两项研究显露,一些痤疮丙酸杆菌噬菌体只裂解与痤疮相关和不相关(但或者是潜在的致病型)的痤疮丙酸杆菌亚型,而对健康皮肤相关的亚型无效⁵⁷⁻⁶⁰。将噬菌体和健康皮肤相关的痤疮杆菌结合使用,在高效特异替代其他痤疮丙酸杆菌方面有伟大潜力(图2)。
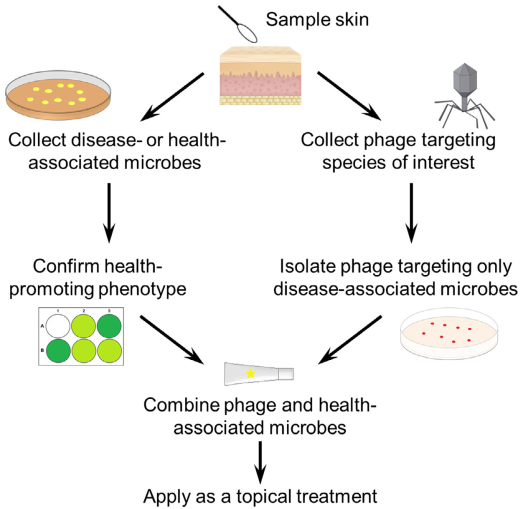
图2 菌群特异性替代的局部外用益生菌结合治疗法。包含有利健康的共生菌以及细菌噬菌体的一种新型局部外用益生菌配方可以靶向性的针对致病菌,并成为高度特异性菌种替代的潜在策略。
银屑病(Psoriasis)
报道显露银屑病患者皮肤微生物菌群的改变或者会诱导Th17通路的激活⁶¹ ⁶²。比拟于未受银屑病侵袭的正常皮肤,病变皮肤上的微生物菌群多样性呈现出削减的趋势⁶³⁻⁶⁵。好比:银屑病皮损部位的放线菌含量显着低于健康皮肤⁶⁴ ⁶⁵,金..葡萄球菌和酿脓链球菌(Streptococcus pyogenes)在银屑病皮损区也只是微量甚至不存在⁶⁴ ⁶⁵。然而,因为研究数据的缺乏以及分歧的抽样手艺,银屑病与皮肤微生态之间的关系还有好多未解之谜。
迄今为止,固然有证据显露益生菌或者经由削减炎症来施展有益的免疫调控感化⁶⁶ ⁶⁷,但仍缺乏益生菌在银屑病治疗过程中所起感化的相关研究。银屑病患者在口服婴儿双歧杆菌(Bifidobacterium infantis)8周后,其体内炎症C-回响卵白和肿瘤坏死因子-α的水平显著降低,但这是否陪伴着临床症状改善还并不清楚⁶⁷。而在银屑病老鼠模型中,经由口服戊糖乳杆菌(Lactobacillus pentosus)GMNL-77可以降低肿瘤坏死因子-α和IL-23-IL-17促炎细胞因子的水平,并陪伴着其红斑和鳞屑皮损部位的削减⁶⁶。越来越多的研究聚焦于微生物菌群在银屑病治疗中施展的调控感化,这也将对络续成长的免疫治疗学进行增补。
脂溢性皮炎(Seborrhoeic dermatitis)
脂溢性皮炎被认为是对游离脂肪酸的一种炎性响应,游离脂肪酸是皮肤常驻真菌糠秕马拉色菌(Malassezia furfur)【此处是指马拉色菌属(Malassezia sp.)译者注】发生的代谢产品⁶⁸ ⁶⁹。而脂溢性皮炎是否获得改善与酵母菌数量削减相关,但马拉色菌的绝对量与病情的严重水平无关⁷⁰ ⁷¹。病情严重水平能够经由细菌多样性是否削减来进行展望⁷²⁻⁷⁴。基于此,微生物制剂被用于治疗脂溢性皮炎。在一项双盲实验中(60位患者),局部外用线状透亮颤菌可以改善患者皮肤红疹,鳞屑以及瘙痒等症状⁷⁰。线状透亮颤菌的裂解物经由树突细胞以及增加调节性T细胞活性促进IL-10的发生⁷⁶。口服副干酪乳杆菌的患者,其头皮屑、红疹以及皮脂溢显著削减,临床症状获得显着缓解⁷⁷。与透亮颤菌雷同,副干酪乳杆菌是经由发生IL-10和转化生长因子-β诱导转换为正常免疫状况⁷⁸。以上究竟均支撑将局部外用或口服益生菌应用于脂溢性皮炎的治疗中。
创面愈合(Wound healing)
皮肤微生物菌群失调以及持续性的炎症都邑导致创面难以愈合⁷⁹。益生菌或者经由调节炎性响应和限制致病菌定植来促进伤口愈合。关于动物实验的最新调集剖析究竟显露,局部外用包含短乳杆菌(Lactobacillus brevis)、植物乳杆菌(Lactobacillus plantarum)和发酵乳酸杆菌的微生物制剂,会导致炎症减轻及加快创面收缩⁸⁰。而人体实验也有报道局部外用益生菌对慢性溃疡有优点:局部外用含有植物乳杆菌的益生菌可以经由调控IL-8和招募吞噬细胞以及成纤维细胞降低菌载量,促进糖尿病溃疡的愈合⁸¹;与此雷同,当非糖尿病患者一连局部外用含植物乳杆菌的微生物制剂30天后,其慢性腿部溃疡有跨越90%的区域获得缓解⁸¹;而口服含乳杆菌的微生物制剂,在治疗慢性糖尿病溃疡方面结果也很显著,其溃疡面积以及炎性标记物的水平均有所降低⁸²。
益生菌的研究究竟也施展在提高烧伤病人的创面愈合能力上⁸³ ⁸⁴,并可以防治皮肤传染。在老鼠模型实验中,局部外用含有植物乳杆菌的微生物制剂可以削减烧伤创面由铜绿假单胞菌(Pseudomonas aeruginosa)引起的皮肤传染⁸⁵。对人类而言,将植物乳杆菌局部用于2度和3度烧伤患者患处,在降低菌载量以及传染风险方面,其能力与磺胺嘧啶银相当,并促进肉芽组织形成和伤口愈合⁸⁴。因为皮肤共生菌对皮肤情况的适应能力强,是以也是局部外用益生菌的优良候选者。好比,皮肤共生菌Propioniferax innocua可以降解形成的生物膜⁸⁶。在老鼠模型中,山羊葡萄球菌(Staphylococcus caprae)对于抗甲氧西林的金..葡萄球菌具有抗菌能力,并能按捺金..葡萄球菌的定植⁸⁷。表皮葡萄球菌发生的抗菌化合物可以选择性的杀伤金..葡萄球菌和酿脓链球菌,同时也能按捺由脂磷壁酸引起的皮肤炎症¹⁰ ¹² ⁸⁸。有两篇文献报道,在具有开放创面的老鼠模型中,痤疮丙酸杆菌可以发生丙酸,以按捺金..葡萄球菌生长⁹⁰。
皮肤癌(Skin cancer)
皮肤微生物菌群失调现象存在于某些皮肤肿瘤中,并促进皮肤癌变。例如,经由葡萄球菌引起的上层抗原的潜在感化,说明了金..葡萄球菌传染与皮肤T细胞淋巴瘤的严重水平之间的关联⁹¹⁻⁹⁴。
相反,健康的皮肤微生物菌群能够经由调节免疫系统以及激活抗肿瘤的免疫监管途径来按捺致癌因子的释放³ ⁹⁵。例如,口服脂磷壁酸(从乳酸杆菌中提取)可以降低皮肤的紫外伤害以及患皮肤癌的风险⁹⁶。比来发现,表皮葡萄球菌发生的碱基分子可以选择性按捺肿瘤细胞的增殖。在老鼠模型中,这些菌的应用也能降低由紫外伤害诱发的肿瘤⁹⁷。健康微生物菌群也能经由调节肿瘤细胞的微生态情况,潜在影响癌症的响应和治疗。研究报道,比拟于无菌或抗菌处理的小鼠模型,在拥有健康肠道微生物菌群的小鼠中应用寡核苷酸免疫疗法和铂化疗手段,肿瘤治疗的结果更佳⁹⁸。是以,我们有来由相信使用益生菌对降低患肿瘤风险以及在皮肤肿瘤的治疗中都是有效的。
结论与瞻望
总之,微生物菌群在皮肤病学中施展着主要的感化,在治疗皮肤病方面也具有伟大潜能。微生物制剂可经由减缓炎症,竖立免疫均衡,阻止致病菌定植来促进健康微生态形成。跟着益生菌的医药用途迅猛增加,其平安性相关的数据还较为缺乏。同时,在免疫低下的人群中,益生菌是否会激发传染或其他严重副感化也值得考虑⁹⁹。是以,在治疗疾病时,需要将微生物菌群作为风险身分做更多的根蒂和风行病学研究。在关于口服和局部外用益生菌的临床试验中,应加大样本量评估其平安性,筛选最佳特定菌种组合、菌种浓度及治疗时间。益生菌与噬菌体或许抗生素连系治疗法或者会成为微生物菌群替代策略的将来成长偏向。
参考文献
1 Bron PA, van Baarlen P, Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol 2011; 10:66–78.
2 Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut–brain–skin axis: from anecdote to translational medicine. Benef Microbes 2014; 5:185–99.
3 Yu Y, Champer J, Beynet D et al. The role of the cutaneous microbiome in skin cancer: lessons learned from the gut. J Drugs Dermatol 2015; 14:461–5.
4 Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet 2012; 13:260–70.
5 Nelson MH, Diven MA, Huff LW et al. Harnessing the microbiome to enhance cancer immunotherapy. J Immunol Res 2015; 2015:368736.
6 Food and Agricultural Organization of the United Nations and World Health Organization. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Available at: http://www.fao.org/tempref/docrep/fao/ meeting/009/y6398e.pdf (last accessed 12 June 2019).
7 Grice EA, Kong HH, Conlan S et al. Topographical and temporal diversity of the human skin microbiome. Science 2009; 324:1190–2.
8 Al-Ghazzewi FH, Tester RF. Impact of prebiotics and probiotics on skin health. Benef Microbes 2014; 5:99–107.
9 Krutmann J. Pre- and probiotics for human skin. Clin Plast Surg 2012; 39:59–64.
10 Christensen GJ, Bruggemann H. Bacterial skin commensals and their role as host guardians. Benef Microbes 2014; 5:201–15.
11 Lai Y, Cogen AL, Radek KA et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol 2010; 130:2211–21.
12 Lai Y, Di Nardo A, Nakatsuji T et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med 2009; 15:1377–82.
13 Palm NW, de Zoete MR, Flavell RA. Immune-microbiota interactions in health and disease. Clin Immunol 2015; 159:122–7.
14 Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer 2013; 13:800–12.
15 Zhong L, Zhang X, Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J Gastroenterol 2014; 20:7878–86.
16 von Hertzen LC, Joensuu H, Haahtela T. Microbial deprivation, inflammation and cancer. Cancer Metastasis Rev 2011; 30:211–23.
17 Geuking MB, Cahenzli J, Lawson MA et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 2011; 34:794–806.
18 Cheng M, Qian L, Shen G et al. Microbiota modulate tumoral immune surveillance in lung through a cdT17 immune cell dependent mechanism. Cancer Res 2014; 74:4030–41.
19 Abrahamsson TR, Jakobsson HE, Andersson AF et al. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol 2012; 129:434–40.
20 Parodi A, Paolino S, Greco A et al. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol 2008; 6:759–64.
21 Williams MR, Gallo RL. The role of the skin microbiome in atopic dermatitis. Curr Allergy Asthma Rep 2015; 15:65.
22 Bjerre RD, Bandier J, Skov L et al. The role of the skin microbiome in atopic dermatitis: a systematic review. Br J Dermatol 2017; 177:1272–8.
23 Baviera G, Leoni MC, Capra L et al. Microbiota in healthy skin and in atopic eczema. Biomed Res Int 2014; 2014:436921.
24 Kong HH, Oh J, Deming C et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 2012; 22:850–9.
25 Clausen ML, Agner T, Lilje B et al. Association of disease severity with skin microbiome and filaggrin gene mutations in adult atopic dermatitis. JAMA Dermatol 2018; 154:293–300.
26 Byrd AL, Deming C, Cassidy SKB et al. Staphylococcus aureus and Staphylococcus epidermidis strain diversity underlying pediatric atopic dermatitis. Sci Transl Med 2017; 9:eaal4651.
27 Orivuori L, Mustonen K, de Goffau MC et al. High level of fecal calprotectin at age 2 months as a marker of intestinal inflammation predicts atopic dermatitis and asthma by age 6. Clin Exp Allergy 2015; 45:928–39.
28 Huang R, Ning H, Shen M et al. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol 2017; 7:392.
29 Chang YS, Trivedi MK, Jha A et al. Synbiotics for prevention and treatment of atopic dermatitis: a meta-analysis of randomized clinical trials. JAMA Pediatr 2016; 170:236–42.
30 Panduru M, Panduru NM, Salavastru CM et al. Probiotics and primary prevention of atopic dermatitis: a meta-analysis of randomized controlled studies. J Eur Acad Dermatol Venereol 2015; 29:232–42.
31 Navarro-Lopez V, Ramirez-Bosca A, Ramon-Vidal D et al. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: a randomized clinical trial. JAMA Dermatol 2018; 154:37–43.
32 Folster-Holst R, Muller F, Schnopp N et al. Prospective, randomized controlled trial on Lactobacillus rhamnosus in infants with moderate to severe atopic dermatitis. Br J Dermatol 2006; 155:1256–61.
33 Gruber C, Wendt M, Sulser C et al. Randomized, placebo-controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Allergy 2007; 62:1270–6.
34 Damm JA, Smith B, Greisen G et al. The influence of probiotics for preterm neonates on the incidence of atopic dermatitis - results from a historically controlled cohort study. Arch Dermatol Res 2017; 309:259–64.
35 Rosenfeldt V, Benfeldt E, Nielsen SD et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol 2003; 111:389–95.
36 Wu YJ, Wu WF, Hung CW et al. Evaluation of efficacy and safety of Lactobacillus rhamnosus in children aged 4–48 months with atopic dermatitis: an 8-week, double-blind, randomized, placebo-controlled study. J Microbiol Immunol Infect 2017; 50:684–92.
37 Nakatsuji T, Chen TH, Narala S et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 2017; 9:eaah4680.
38 Nakatsuji T, Yun T, Butcher A et al. Clinical improvement in atopic dermatitis following autologous application of microbiome therapy targeting Staphylococcus aureus. J Invest Dermatol 2018; 138 (5 Suppl.):S72.
39 Myles IA, Earland NJ, Anderson ED et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 2018; 3:120608.
40 Blanchet-Rethore S, Bourdes V, Mercenier A et al. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin Cosmet Invest Dermatol 2017; 10:249–57.
41 Di Marzio L, Centi C, Cinque B et al. Effect of the lactic acid bacterium Streptococcus thermophilus on stratum corneum ceramide levels and signs and symptoms of atopic dermatitis patients. Exp Dermatol 2003; 12:615–20.
42 Gueniche A, Knaudt B, Schuck E et al. Effects of nonpathogenic gram-negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: a prospective, randomized, double-blind, placebo-controlled clinical study. Br J Dermatol 2008; 159:1357–63.
43 Jung GW, Tse JE, Guiha I et al. Prospective, randomized, open label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J Cutan Med Surg 2013; 17:114–22.
44 Kim J, Ko Y, Park YK et al. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition 2010; 26:902–9.
45 Fabbrocini G, Bertona M, Picazo O et al. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef Microbes 2016; 7:625–30.
46 Yu Y, Champer J, Garban H et al. Typing of Propionibacterium acnes: a review of methods and comparative analysis. Br J Dermatol 2015; 172:1204–9.
47 McDowell A, Nagy I, Magyari M et al. The opportunistic pathogen Propionibacterium acnes: insights into typing, human disease, clonal diversification and CAMP factor evolution. PLOS ONE 2013; 8: e70897.
48 Scholz CF, Jensen A, Lomholt HB et al. A novel high-resolution single locus sequence typing scheme for mixed populations of Propionibacterium acnes in vivo. PLOS ONE 2014; 9:e104199.
49 Fitz-Gibbon S, Tomida S, Chiu BH et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol 2013; 133:2152–60.
50 Tomida S, Nguyen L, Chiu BH et al. Pan-genome and comparative genome analyses of Propionibacterium acnes reveal its genomic diversity in the healthy and diseased human skin microbiome. MBio 2013; 4:e00003–13.
51 Yu Y, Champer J, Agak GW et al. Different Propionibacterium acnes phylotypes induce distinct immune responses and express unique surface and secreted proteomes. J Invest Dermatol 2016; 136:2221–8.
52 Kober MM, Bowe WP. The effect of probiotics on immune regulation, acne, and photoaging. Int J Womens Dermatol 2015; 1:85–9.
53 Kang BS, Seo JG, Lee GS et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J Microbiol 2009; 47:101–9.
54 AOBiome Therapeutics. AOBiome Therapeutics reports positive efficacy results from phase 2b clinical trial of ammonia oxidizing bacteria (AOB) for the treatment of acne vulgaris. Available at: https://www.aobiome.com/pressreleases/aobiome-therapeuticsreports-positive-efficacy-results-from-phase-2b-clinical-trial-of-ammonia-oxidizing-bacteria-aob-for-the-treatment-of-acne-vulgaris (last accessed 12 June 2019).
55 Nodake Y, Matsumoto S, Miura R et al. Pilot study on novel skin care method by augmentation with Staphylococcus epidermidis, an autologous skin microbe – a blinded randomized clinical trial. J Dermatol Sci 2015; 79:119–26.
56 Wang Y, Kuo S, Shu M et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol 2014; 98:411–24.
57 Liu J, Yan R, Zhong Q et al. The diversity and host interactions of Propionibacterium acnes bacteriophages on human skin. ISME J 2015; 9:2078–93.
58 Marinelli LJ, Fitz-Gibbon S, Hayes C et al. Propionibacterium acnes bacteriophages display limited genetic diversity and broad killing activity against bacterial skin isolates. MBio 2012; 3:e00279–12.
59 Brown TL, Petrovski S, Dyson ZA et al. The formulation of bacteriophage in a semi solid preparation for control of Propionibacterium acnes growth. PLOS ONE 2016; 11:e0151184.
60 Jonczyk-Matysiak E, Weber-Dabrowska B, Zaczek M et al. Prospects of phage application in the treatment of acne caused by Propionibacterium acnes. Front Microbiol 2017; 8:164.
61 Fry L, Baker BS, Powles AV et al. Is chronic plaque psoriasis triggered by microbiota in the skin? Br J Dermatol 2013; 169:47–52.
62 Martin DA, Towne JE, Kricorian G et al. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol 2013; 133:17–26.
63 Alekseyenko AV, Perez-Perez GI, De Souza A et al. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome 2013; 1:31.
64 Fahlen A, Engstrand L, Baker BS et al. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res 2012; 304:15–22.
65 Gao Z, Tseng CH, Strober BE et al. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLOS ONE 2008; 3: e2719.
66 Chen YH, Wu CS, Chao YH et al. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J Food Drug Anal 2017; 25:559–66.
67 Groeger D, O’Mahony L, Murphy EF et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013; 4:325–39.
68 Lee YW, Lee SY, Lee Y et al. Evaluation of expression of lipases and phospholipases of Malassezia restricta in patients with seborrheic dermatitis. Ann Dermatol 2013; 25:310–14.
69 Sparber F, LeibundGut-Landmann S. Host responses to Malassezia spp. in the mammalian skin. Front Immunol 2017; 8:1614.
70 Gupta AK, Batra R, Bluhm R et al. Skin diseases associated with Malassezia species. J Am Acad Dermatol 2004; 51:785–98.
71 Pierard GE, Arrese JE, Pierard-Franchimont C et al. Prolonged effects of antidandruff shampoos - time to recurrence of Malassezia ovalis colonization of skin. Int J Cosmet Sci 1997; 19:111–17.
72 Xu Z, Wang Z, Yuan C et al. Dandruff is associated with the conjoined interactions between host and microorganisms. Sci Rep 2016; 6:24877.
73 Park T, Kim HJ, Myeong NR et al. Collapse of human scalp microbiome network in dandruff and seborrhoeic dermatitis. Exp Dermatol 2017; 26:835–8.
74 Tanaka A, Cho O, Saito C et al. Comprehensive pyrosequencing analysis of the bacterial microbiota of the skin of patients with seborrheic dermatitis. Microbiol Immunol 2016; 60:521–6.
75 Gueniche A, Cathelineau AC, Bastien P et al. Vitreoscilla filiformis biomass improves seborrheic dermatitis. J Eur Acad Dermatol Venereol 2008; 22:1014–15.
76 Volz T, Skabytska Y, Guenova E et al. Nonpathogenic bacteria alleviating atopic dermatitis inflammation induce IL-10-producing dendritic cells and regulatory Tr1 cells. J Invest Dermatol 2014; 134:96–104.
77 Reygagne P, Bastien P, Couavoux MP et al. The positive benefit of Lactobacillus paracasei NCC2461 ST11 in healthy volunteers with moderate to severe dandruff. Benef Microbes 2017; 8:671–80.
78 von der Weid T, Bulliard C, Schiffrin EJ. Induction by a lactic acid bacterium of a population of CD4+ T cells with low proliferative capacity that produce transforming growth factor b and interleukin-10. Clin Diagn Lab Immunol 2001; 8:695–701.
79 Canesso MC, Vieira AT, Castro TB et al. Skin wound healing is accelerated and scarless in the absence of commensal microbiota. J Immunol 2014; 193:5171–80.
80 Tsiouris CG, Kelesi M, Vasilopoulos G et al. The efficacy of probiotics as pharmacological treatment of cutaneous wounds: meta-analysis of animal studies. Eur J Pharm Sci 2017; 104:230–9.
81 Peral MC, Rachid MM, Gobbato NM et al. Interleukin-8 production by polymorphonuclear leukocytes from patients with chronic infected leg ulcers treated with Lactobacillus plantarum. Clin Microbiol Infect 2010; 16:281–6.
82 Mohseni S, Bayani M, Bahmani F et al. The beneficial effects of probiotic administration on wound healing and metabolic status in patients with diabetic foot ulcer: a randomized, double-blind, placebo-controlled trial. Diabetes Metab Res Rev 2019; DOI: https:// doi.org/10.1002/dmrr.2970
83 El-Ghazely MH, Mahmoud WH, Atia MA, Eldip EM. Effect of probiotic administration in the therapy of pediatric thermal burn. Ann Burns Fire Disasters 2016; 29:268–72.
84 Peral MC, Martinez MA, Valdez JC. Bacteriotherapy with Lactobacillus plantarum in burns. Int Wound J 2009; 6:73–81.
85 Valdez JC, Peral MC, Rachid M et al. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: the potential use of probiotics in wound treatment. Clin Microbiol Infect 2005; 11:472–9.
86 Lopes EG, Moreira DA, Gullon P et al. Topical application of probiotics in skin: adhesion, antimicrobial and antibiofilm in vitro assays. J Appl Microbiol 2017; 122:450–61.
87 Paharik AE, Parlet CP, Chung N et al. Coagulase-negative staphylococcal strain prevents Staphylococcus aureus colonization and skin infection by blocking quorum sensing. Cell Host Microbe 2017; 22:746–56.
88 Cogen AL, Yamasaki K, Sanchez KM et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol 2010; 130:192–200.
89 Shu M, Wang Y, Yu J et al. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLOS ONE 2013; 8:e55380.
90 Wang Y, Dai A, Huang S et al. Propionic acid and its esterified derivative suppress the growth of methicillin-resistant Staphylococcus aureus USA300. Benef Microbes 2014; 5:161–8.
91 Mirvish JJ, Pomerantz RG, Falo LD Jr et al. Role of infectious agents in cutaneous T-cell lymphoma: facts and controversies. Clin Dermatol 2013; 31:423–31.
92 Nguyen V, Huggins RH, Lertsburapa T et al. Cutaneous T-cell lymphoma and Staphylococcus aureus colonization. J Am Acad Dermatol 2008; 59:949–52.
93 Jackow CM, Cather JC, Hearne V et al. Association of erythrodermic cutaneous T-cell lymphoma, superantigen-positive Staphylococcus aureus, and oligoclonal T-cell receptor Vb gene expansion. Blood 1997; 89:32–40.
94 Tokura Y, Yagi H, Ohshima A et al. Cutaneous colonization with staphylococci influences the disease activity of Sezary syndrome: a potential role for bacterial superantigens. Br J Dermatol 1995; 133:6–12.
95 Chen YE, Tsao H. The skin microbiome: current perspectives and future challenges. J Am Acad Dermatol 2013; 69:143–55.
96 Weill FS, Cela EM, Paz ML et al. Lipoteichoic acid from Lactobacillus rhamnosus GG as an oral photoprotective agent against UV-induced carcinogenesis. Br J Nutr 2013; 109:457–66.
97 Nakatsuji T, Chen TH, Butcher AM et al. A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Sci Adv 2018; 4:eaao4502.
98 Iida N, Dzutsev A, Stewart CA et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013; 342:967–70.
99 Didari T, Solki S, Mozaffari S et al. A systematic review of the safety of probiotics. Expert Opin Drug Saf 2014; 13:227–39.
100 van de Wijgert JH, Jespers V. Incorporating microbiota data into epidemiologic models: examples from vaginal microbiota research. Ann Epidemiol 2016; 26:360–5.

大家好,小乐今天来为大家解答qq好友管理在哪里以下问题,qq好友管理app很多人还不知道,现在让我们一起来看看吧!1、在打开的QQ主界面,右键点
大家好,小乐今天来为大家解答工作日程安排软件以下问题,工作日程表软件很多人还不知道,现在让我们一起来看看吧!1、优秀的时间管理工具
大家好,小丽今天来为大家解答毒网电影中文以下问题,毒网电影中文很多人还不知道,现在让我们一起来看看吧!1、边境小城吉川市,发生贩卖毒
大家好,小美今天来为大家解答应收账款分录以下问题,收回前期已核销的应收账款分录很多人还不知道,现在让我们一起来看看吧!1、销售商品时
大家好,小美今天来为大家解答吉田优希以下问题,吉田友一很多人还不知道,现在让我们一起来看看吧!1、姓名:吉田有希艺名:YUUKI生日:1989年
迎接人人积极投稿,末学急需材料,投稿邮箱:nzjsb2020@163.com三年了,一千多个日夜,终于,我再次可以感触到早晨平坦的阳光下,轻风拂过面颊的惬
大家好,小乐今天来为大家解答是否享受一补怎么填以下问题,是否享受生活补助怎么填很多人还不知道,现在让我们一起来看看吧!1、法律分析:
传统小分子药物的药理感化是一药对应一靶标,即使一药多靶 (包罗脱靶)也是1:1的离别连系。药物与靶标的连系遵循质量感化定律,连系的热力学和
本文内容来自网友供稿,如有信息侵犯了您的权益,请联系反馈核实
Copyright 2024.爱妻自媒体,让大家了解更多图文资讯!